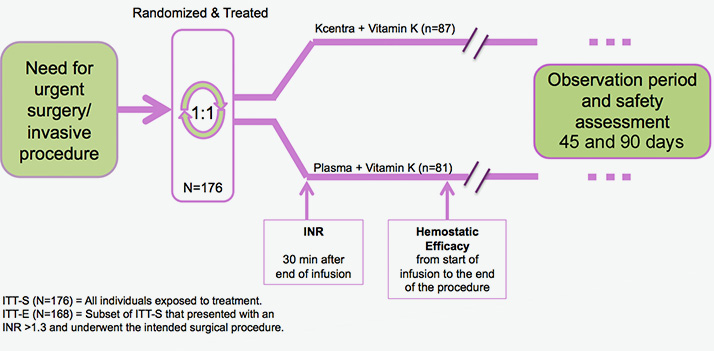
urgent surgery/invasive procedures
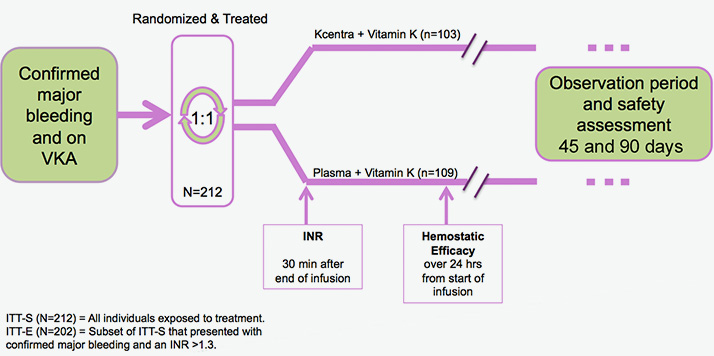
Prospective, randomized, open-label, active-controlled, multicenter, noninferiority trial2
Explore Kcentra data vs plasma for urgent warfarin reversal in 2 head-to-head trials
| Efficacy endpoint | Urgent Surgery/Invasive Procedures trial | Acute Major Bleeding trial |
|---|---|---|
| Effective hemostasis | Kcentra superior | Kcentra and plasma equally effective |
| Early INR reduction | Kcentra superior | Kcentra superior |
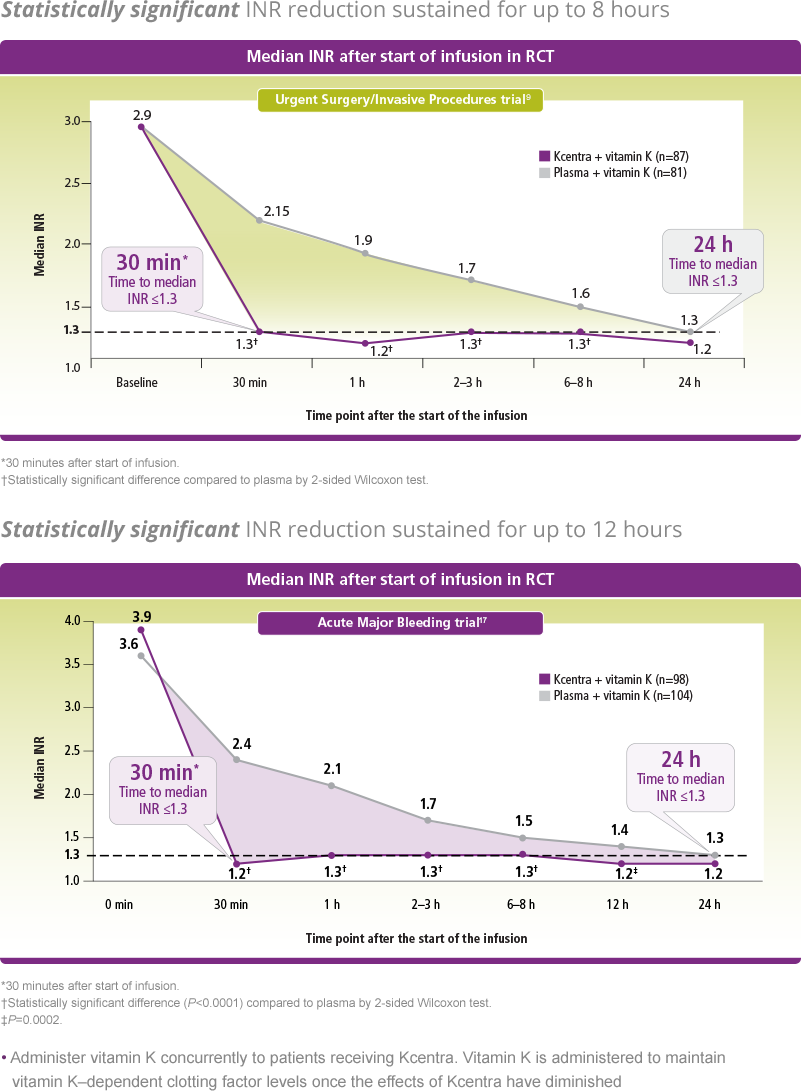
Effective hemostasis measured up to 24 hours for the Acute Major Bleeding trial and until the end of procedure (up to 24 hours) for the Urgent Surgery/Invasive Procedures trial. Rapid INR reduction to ≤1.3 at 0.5 hours after end of infusion.
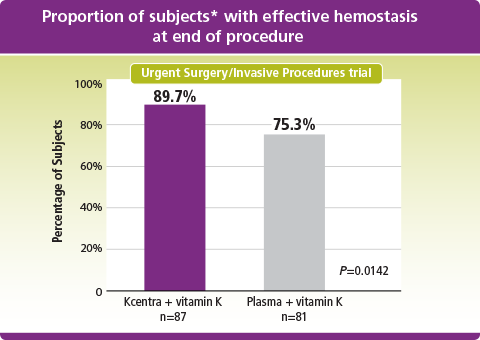
Kcentra–plasma (%) [95% CI] = 14.3 [2.8, 25.8] (prespecified noninferiority margin >–10%; Kcentra superior to plasma as lower limit of 95% CI >0).
*Intent to treat-efficacy (ITT-E) population.
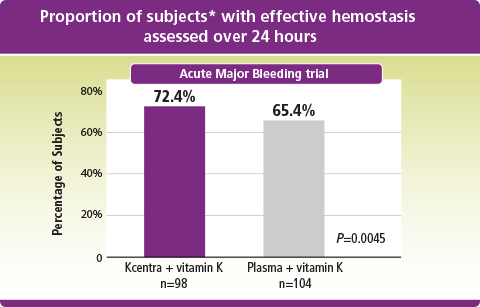
Kcentra–plasma (%) [95% CI] = 7.1 [–5.8, 19.9] (prespecified noninferiority margin >–10%). Farrington–Manning P value for noninferiority, rejecting null hypothesis of inferiority of 4F-PCC.
*ITT-E population
Prospective, randomized, open-label, active-controlled, multicenter, noninferiority trial2

Prospective, randomized, open-label, active-controlled, multicenter, noninferiority trial10
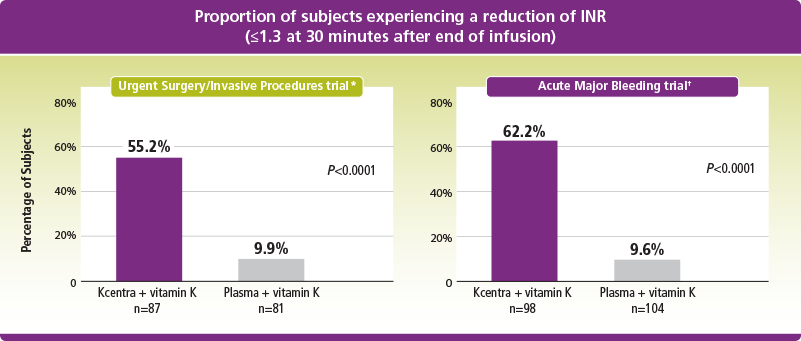
*Kcentra—plasma (%) [95% CI] = 45.3 [31.9, 56.4] for noninferiority (prespecified noninferiority margin >–10% in the Urgent Surgery/Invasive Procedures trial; Kcentra superior to plasma if lower limit 95% CI >0).
†Kcentra—plasma (%) [95% CI] = 52.6 [39.4, 65.9] for noninferiority (prespecified noninferiority margin >–10%; Kcentra superior to plasma if lower limit 95% CI >0) in the Acute Major Bleeding trial.