Single-dose administration and simple dosage calculator
Dosage calculator
Quickly calculate the recommended dose for your patient
Enter the patient's body weight below and select the appropriate pretreatment INR:

YES | NO


Add this calculator to your smartphone’s home screen
Save a link on your smartphone or tablet home screen for faster access.
iPhone is a registered trademark of Apple Inc.
Android is a trademark of Google Inc.
- Tap the
 button
button - Tap "Add to Home Screen"
- Tap the
 button
button - Tap "Add to Home Screen"
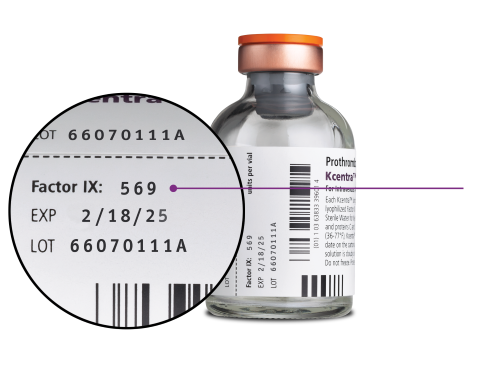
Kcentra dose is calculated using Factor IX content
Kcentra is available in 2 vial sizes for added convenience
- 500 units for use with 20-mL vial of Sterile Water for Injection, USP
- 1000 units for use with 40-mL vial of Sterile Water for injection, USP
How to calculate dosage
A single dose of Kcentra is determined by the patient’s pretreatment INR and weight
| Pretreatment INR | 2–<4 | 4–6 | >6 |
|---|---|---|---|
| Dose* of Kcentra (units† of Factor IX)/kg body weight | 25 | 35 | 50 |
| Maximum dose‡ (units of Factor IX) | Not to exceed 2500 | Not to exceed 3500 | Not to exceed 5000 |
*Dosing is based on body weight. Dose based on actual potency is stated on the vial, which will vary from 20–31 Factor IX units/mL after reconstitution. The actual potency for 500 unit vial ranges from 400–620 units/vial. The actual potency for 1000 unit vial ranges from 800–1240 units/vial.
†Units refer to international units.
‡Dose is based on body weight up to but not exceeding 100 kg. For patients weighing more than 100 kg, maximum dose should not be exceeded.
- Repeat dosing is not supported by clinical data and is not recommended
-
Administer Kcentra:
- By intravenous infusion at a rate of 0.12 mL/kg/min (~3 units/kg/min) up to a maximum rate of 8.4 mL/min
- Concurrently with vitamin K
- Through a separate infusion line
