Choose Kcentra instead of fresh frozen plasma for speed with less volume
Shorter time to surgery with Kcentra vs plasma
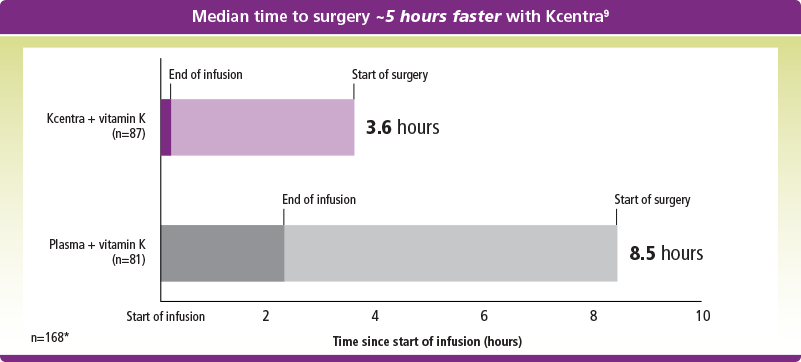
*ITT-E population.
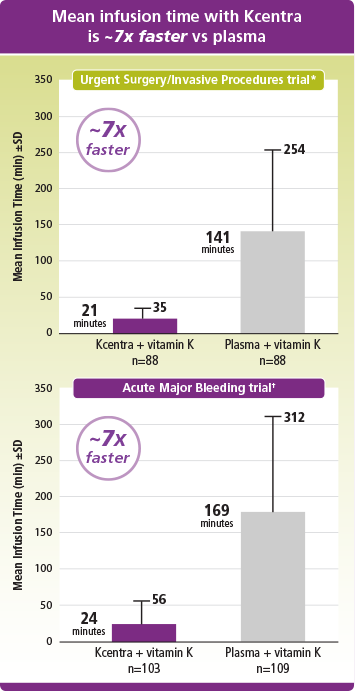
Faster mean infusion time with Kcentra vs plasma
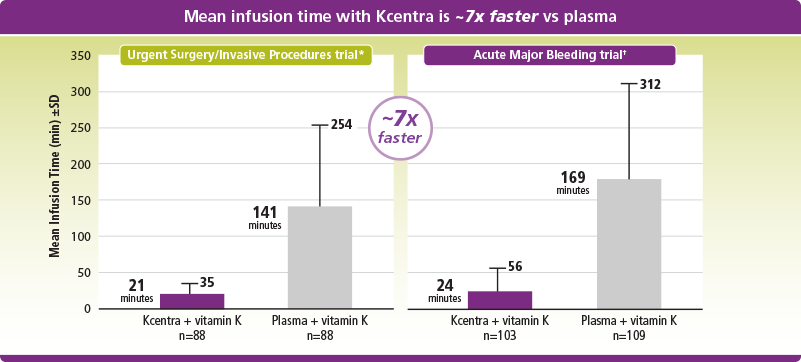
*Mean infusion time was 21 min (±14 min) for Kcentra and 141 min (±113 min) for plasma.
†Mean infusion time was 24 min (±32 min) for Kcentra and 169 min (±143 min) for plasma.
- No need for thawing or ABO typing
Kcentra uses less volume and is more concentrated than plasma
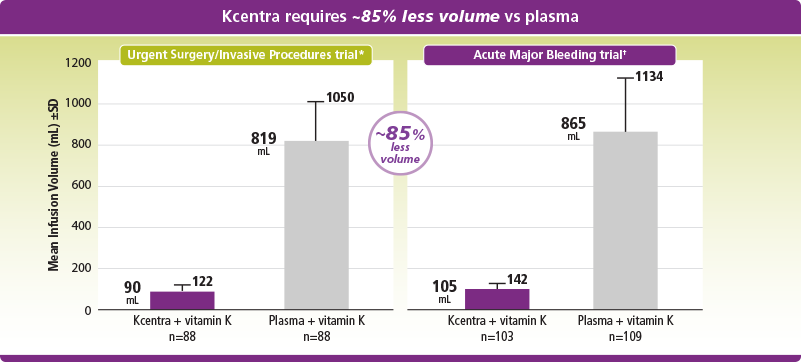
*Mean infusion volume was 90 mL (±32 mL) for Kcentra and 819 mL (±231 mL) for plasma.
†Mean infusion volume was 105 mL (±37 mL) for Kcentra and 865 mL (±269 mL) for plasma.
- 3 vials of Kcentra (an average volume of 2500 IU administered as a single dose) are equal to 10–12 units of plasma

Fresh frozen plasma use is associated with unique risks and challenges
32% of reported fatalities have occurred as a result of TACO, which results from cardiogenic (hydrostatic) pulmonary edema
26% of reported fatalities have occurred as a result of TRALI or possible TRALI, which results from noncardiogenic (permeability) pulmonary edema
- Both TACO and TRALI are likely underdiagnosed and underreported18
Prolonged length of hospital and ICU stay
Significant increases in hospitalization costs per visit
TACO=transfusion-associated circulatory overload
TRALI=transfusion-related acute lung injury
*Based on the cited references, fatality-related data listed here are attributable to the use of blood components and are not specific to the use of plasma.